Introduction: For almost 50 years,induction therapy (IT) is the standard of care for acute myeloid leukemia (AML) patients. Nevertheless, little is known about cardiovascular (CV) toxicity during IT. We aimed to describe CV events (CVE)occurring during IT and to identify clinical, hematological and molecular risk factors of such events.
Methods: Two hundred and twenty-four patients of 18 years or more (median age 61 (51;67) years, 58% males), treated with ITbetween 2014 and 2021 were enrolled in this study. Two hundred and one (90%) received an anthracycline-based IT, the others received gemtuzumab ozogamicin as part of a clinical protocol research. Survival from the time from initiation of IT until death or until the initiation of the second course of therapy (either salvage regimen in case of primary refractory or relapsed AML, or intensive consolidation therapy in case of response) was considered. The primary endpoint was the time to occurrence of the first CVE after IT initiation, with death as a competing event. Multivariate analysis included significant covariate, identified by univariate models among initial characteristics and occurrence of infection (coded as time dependent covariate).
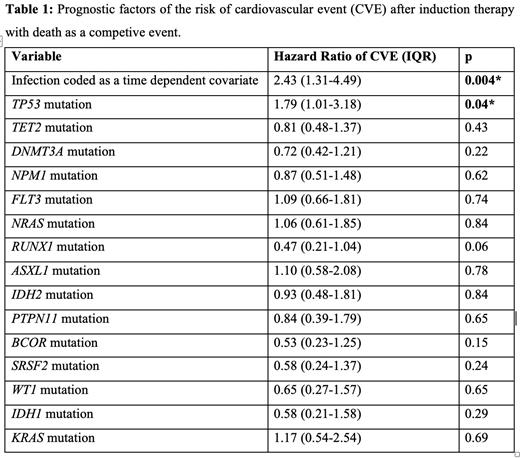
Results: Hematological characteristics were as follows: European LeukemiaNet (ELN) 2022 risk of myelodysplastic neoplasm (MDS)/AML was low, intermediate and adverse in 13%, 29% and 58% of patients respectively. Main mutations encounters were: DNMT3A, FLT3, NPM1, TET2, NRAS, RUNX1, ASXL1, IDH2, PTPN11, BCOR, SRSF2, WT1 and TP53. Main CV risk factors were tobacco (36%), arterial hypertension (35%), obesity (24%), hyperlipidemia (22%), diabetes mellitus (11%). Baseline left ventricular ejection fraction (assessed by transthoracic echocardiography) was >45% in all patients, >55% in 95% of patients. Seventy-five patients (33%) presented a CVE during IT. Eighty-six percent of CVE were severe (grade 3 or more). Patients presenting CVE during IT were significantly older (p=0.02) and presented more often a past history of dilated cardiomyopathy (p=0.04), arterial hypertension (p=0.005) or dyslipidemia (p=0.005). At baseline, CVE patients presented a greater left auricle volume (p=0.005), higher tricuspid regurgitation grade (p=0.01), and more often a right ventricular dilatation or a significative valvopathy (respectively p=0.01 and p=0.03). There was no difference in CVE occurrence between patients treated with and without anthracyclin (p=0.2). Competing risk models identified the following adverse characteristic for the risk of first CVE, namely the occurrence of the first infectious event (Hazard ratio [HR]= 2.43, p=0.005), TP53 mutation (HR=1.79, p=0.04). No other clinical characteristics nor mutation (including TET2 and DNMT3A) were identified. Multivariate analyses demonstrated that TP53 mutation (HR=1.78, p=0.04) and occurrence of infection (HR=2.29, p=0.008) retained independent prognostic value, see Table 1. In a response stratified model, only TP53 mutation was a risk factor of first CVE (HR=2.46, p=0.008).
Conclusions: CVE occurred in 33% of patients during IT and were severe. The major adverse role of onset of infection for CVE occurrence after IT could overcome the prognostic value of mutations usually associated with CV risk in the healthy population (i.e. TET2, DNMT3A, ASXL1, ...). TP53 mutation was the only molecular abnormality associated with CVE, in agreement with the increased risk of doxorubicin-induced cardiac toxicity in a mouse model of TP53 clonal hematopoiesis developed by Sano et al. These results suggest that TP53 mutated patients could benefit from careful CV monitoring and optimization of primary prevention treatment.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal